Department of Natural Sciences
Monitoring of Clinical Trials (MSc)
Lecture
Compulsory Course
- in MSc Biomedical Sciences, 1st semester
- 6h/week (2L/2E/2P)
- Credits: 8 ECTS
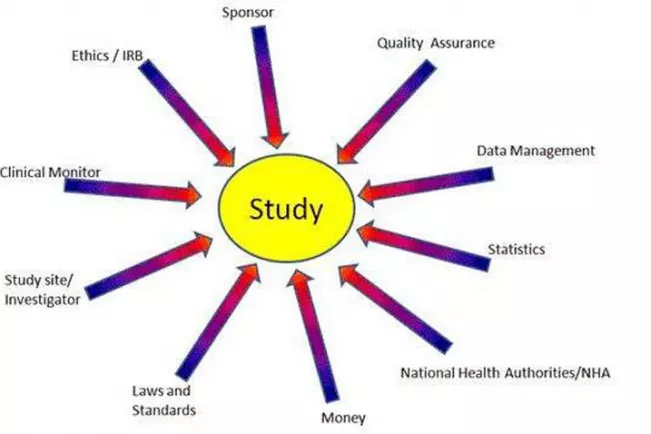
Clinical research and clinical trials are an essential part of an integral part of biomedical innovation. Thereby the size and complexity of the implementation of clinical trials is often unacknowledged. For instance: a clinical trial can include the recruitment of thousands of patients by hundreds of clinical research sites worldwide. This is, of course, is also reflected in the scale and complexity of organizations and processes necessary to conduct a clinical trial. Over the years, studies have become increasingly complex, so the number of employees required is also steadily increasing.
In this course you will get insight in management of multi-center international clinical trials. This includes the project management as well as the aspects of site management or monitoring in drug development and medical devices.
Learning outcomes:
- The lecture, seminars and practical course will provide you with a in depth understanding of management and regulation of clinical trials
- You have a detailed understanding of roles and responsibilities within clinical trial implementation.
- Based on practical exercises you are familiar with the role as clinical research associate or clinical monitor.
- You can also use the module to improve the presentation and project management skills
Content:
- Details on Project Management of clinical studies relevant for the setup, conduct and close-out phase.
- Details of Regulations such as Good clinical practice (GCP), EU Regulation Nr. 536/2014 AMG, CFR 21, ISO 14155
- Analyses and Reporting of clinical trials
Exercises
Content
- Focus of the exercise is the monitoring of clinical trials.
- Regulation regarding monitoring of clinical trials.
- Practical exercise about monitoring of clinical trials
- Activities and responsibilities of CRAs during setup, conduct and close-out phase of clinical trials.
Internship
Content
- Conduct of exercise regarding planning conduct and close out of clinical trials: such as detailed study planning
Requirements
Individual PowerPoint presentations on a topic exploring specific aspects of clinical research and trial monitoring.